| Address | Teheranro 108-gil 22, Gangnam-gu, Seoul, 135-502, Korea | ||
|---|---|---|---|
| Telephone | +82-2-550-1234 | Fax | +82-2-550-1215 |
| Website | www.roche-diagnostics.co.kr | korea.diagnostics@roche.com | |
| Roche Diagnostics Korea is a local affiliate of Diagnostics Division at Roche, a global healthcare group based in Basel. It has provided innovative diagnostics solutions and services for testing bloods and body fluids & tissues, contributing to early detection, prevention, diagnosis, treatment and monitoring of disease. Roche Diagnostics is the world leader in in vitro diagnostics and tissue-based cancer diagnostics, and a front-runner in diabetes management. Roche is a pioneer of Personalized Healthcare and it aims at providing medicines and diagnostic tools that enable tangible improvements in the health, quality of life and survival of patients. | |||




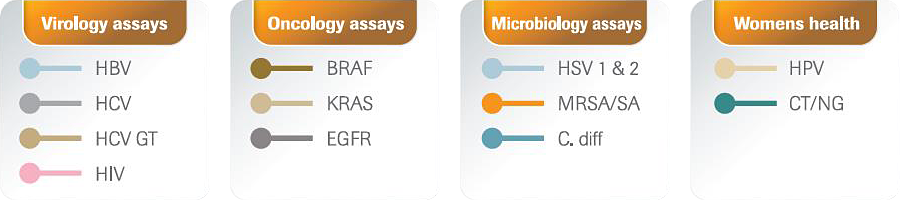
The cobas® 4800 System and the Virology assays deliver absolute versatility with a unique combination of attributes that empowers your laboratory to keep pace with ever changing demands. Broad menu or consolidation can be achieved because of the menu of 12 CE-IVD tests on the system. Efficiency is also gained with the ability to load primary or secondary tubes directly onto the platform and the ready to use reagents. An expanding assay menu reinforces the flexibility offered by the cobas® 4800 System.
An expanding assay menu reinforces the flexibility offered by the cobas® 4800 System.

| Address | 7F, SamTan Bldg. 421, YoungDong-daero, Gangnam-gu, Seoul, 06182, Korea | ||
|---|---|---|---|
| Telephone | +82-2-3429-3500 | Fax | +82-2-3429-3772 |
| Website | www.abbott.com | junpyo.hong@abbott.com | |
| We find out about our mission to make better health possible for humankind. Our heritage has discovered 125-year history of innovation and takes a look at the road ahead. Our diverse businesses enable us to offer vast solutions to people worldwide during every stage of life and enable them to live not just longer but better. Our success and that of the communities we serve are intertwined. Find out how we work to realize our shared potential. | |||





| Address | 6F, Poongsan Bldg. 23, Chungjeong-ro, Seodaemun-gu, Seoul, 120-837, Korea | ||
|---|---|---|---|
| Telephone | +82-2-3450-7880 | Fax | +82-2-3450-7879 |
| Website | www.siemens.co.kr/healthcare | bora.kim@siemens-healthineers.com | |
| Siemens Healthineers is the separately managed healthcare business of Siemens AG enabling healthcare providers worldwide to meet their current challenges and to excel in their respective environments. A leader in medical technology, Siemens Healthineers is constantly innovating its portfolio of products and services in its core areas of diagnostic and therapeutic imaging and in laboratory diagnostics and molecular medicine. Siemens Healthineers is also actively developing its digital health services and enterprise services. To help customers succeed in today’s dynamic healthcare marketplace, Siemens Healthineers is championing new business models that maximize opportunity and minimize risk for healthcare providers. In fiscal 2016, which ended on September 30, 2016, Siemens Healthineers generated revenue of €13.5 billion and profit of over €2.3 billion and has about 46,000 employees worldwide. Further information is available at www.siemens.com/healthineers |
|||




| Address | SPG Building 5F, 166 Jeongjail-ro, Bundang-gu, Seongnam-si, Gyeonggi-do, 13558, Korea | ||
|---|---|---|---|
| Telephone | +82-31-8014-6700 | Fax | +82-31-8014-6799 |
| Website | www.alere.com | alerehealthcare@alere.com | |
| Knowing now matters.™ Alere delivers reliable and actionable information through rapid diagnostic tests, resulting in better clinical and economic healthcare outcomes globally. Our innovative diagnostics deliver reliable and actionable information, resulting in better clinical and economic healthcare outcomes globally. Our socially-conscious business approach focuses on creating breakthrough, cost-effective diagnostic solutions that address the most intractable diseases for all populations in all corners of the world including infectious disease, cardiometabolic disease and toxicology. Alere is committed to delivering high-quality products and services that patients and providers can rely on for consistently accurate and actionable information. We deploy unique innovative technologies that not only transform diagnostic products, but also expand into new platforms and solutions with proven health and economic outcomes. |
|||




| Address | 3F 281 Kwangyeong-ro, Gangnam-gu, Seoul, Korea | ||
|---|---|---|---|
| Telephone | +82-2-6420-3100 | Fax | +82-2-6420-3120 |
| Website | www.beckmancoulter.com | ||
| MOVE HEALTHCARE FORWARD WITH BECKMAN COULTER As healthcare undergoes a major transformation, navigating the paradigm shift is easier with a partner. Join us at LMCE and see for yourself how partnership with Beckman Coulter is helping today’s clinical laboratories prepare for tomorrow. Experience the Beckman Coulter Diagnostics Difference firsthand and find out how an integrated solution of tools, insights, information management systems and continuous process improvements can help your laboratory achieve excellence now and in the future. |
|||




| Address | 132 Teheran-ro, Gangnam-gu, Seoul, 135-755, Korea | ||
|---|---|---|---|
| Telephone | +82-2-527-5114 | Fax | +82-2-527-5004 |
| Website | www.handok.com | Handok@handok.com | |
| HANDOK, a leading innovation-driven pharmaceutical/health-care company in Korea, develops, manufactures and distributes healthcare solutions to improve the health and quality of human life.Handok has a core business focus in diabetes, cardiovascular, muscular skeletal, psychoneurotic disease, human vaccines, medical devices, diagnostics and consumer health. Handok, founded in 1954, grew as a joint venture with Hoechst/Aventis/Sanofi from 1964 to 2012. Handok has also established strategic collaborations in several areas with multiple multinational pharmaceutical companies. |
|||


| Address | 91, Ogeum-ro, Songpa-gu, Seoul, Korea | ||
|---|---|---|---|
| Telephone | +82-2-2240-4000 | Fax | +82-2-2240-4040 |
| Website | www.seegene.com | korea@seegene.com | |
| Seegene is the world's leading developer of multiplex molecular technologies and multiplex clinical molecular diagnostics (MDx). Seegene's core enabling technologies - DPO™, TOCE™ and MuDT™ - are the foundation for MDx tests that can simultaneously detect and quantitate multiple targets with high sensitivity, specificity and reproducibility. Seegene offers MDx tests in infectious diseases, genetics, drug resistance, and oncology, and actively partners in the development of new tests and the improvement of the quality of healthcare utilizing its breakthrough multiplex molecular technologies. For more information about Seegene, click here. |
|||

| Address | 3F, Seoul International Tower, 203, Teheran-ro, Gangnam-gu, Seoul, 06141, Korea | ||
|---|---|---|---|
| Telephone | +82-2-3498-5300 | Fax | +82-2-3498-5312 |
| Website | www.sysmex.co.kr | khjo@sysmex.co.kr | |
| Sysmex Corporation is an integrated company, developing and manufacturing the instruments, reagents and software that are needed for in vitro diagnostics, along with the necessary sales and support networks. Serving customers for more than 40 years, Sysmex focuses on technological leadership in in vitro diagnostics (IVD) and information that make a difference in the health of people worldwide. The company is also exploring emerging opportunities in the life science field. Its R&D efforts focus on the development of high-value-added testing and diagnostic technologies that are innovative, original and optimize individual health and personalized healthcare. Sysmex also seeks to leverage its state-of-the-art technologies for cell, gene and protein analysis. |
|||




| Address | Yoosung Btd. 1,2,7F, 121, Yeoksam-ro, Gangnam-gu, Seoul, 06243, Korea | ||
|---|---|---|---|
| Telephone | +82-2-2188-4700 | Fax | +82-2-539-7748 |
| Website | www.biomerieux.co.kr | inhwa.jeong@biomerieux.com | |
| A world leader in the field of in vitro diagnostics for 50 years, bioMérieux provides diagnostic solutions (reagents, instruments, software and services) which determine the source of disease and contamination to improve patient health and ensure product safety. We develop tests that bring high medical value for clinical decisions in the areas of infectious diseases, cardiovascular emergencies and targeted cancers. bioMérieux is determined to continue to play a pioneering role by innovating and designing the diagnostics of the future to address the major challenges for public health worldwide. |
|||



| Address | 107, Ihyeon-ro 30beon-gil, Giheung-gu, Yongin-si, Gyeonggi-do,446-770, Korea | ||
|---|---|---|---|
| Telephone | +82-10-8987-7593 | Fax | +82-31-260-9416 |
| Website | www.greencrossms.com | ||
| In 1987, Green Cross Medical Science Corp. started as the first-generation company in Korea in IVD field with launching GENEDIA HIV 1/2 ELISA test kit which diagnose AIDS, and has been expanding its business to blood bag, Hemodialysis solution, Blood glucose monitoring system. We are currently upgrading IVD portfolio through development of Real-Time PCR reagent and specific recombinant antibody production technology.Green Cross Medical Science Corp. is focusing to be ‘Global Company’ with “Open R&D / Innovation” to strengthen marketing and development of market-demand products. |
|||



| Address | LG Gwanghwamun Bldg. 58, Saemunan-ro, Jongno-gu, Seoul, 03184, Korea | ||
|---|---|---|---|
| Telephone | +82-80-023-5757 | Fax | +82-2-3773-2100 |
| Website | www.lgchem.com/kr | lgivd@lgchem.com | |
| LG Chem has been developing molecular diagnostics, immunodiagnostics and allergic diagnostic products, including Koreas' first hepatitis C diagnostic reagent, through ceaseless investment and R&D for the last 20 years to secure core competence in the In-Vitro Diagnostic business. We focus on finding the target materials needed for early diagnosis of diseases with excellent domestic and foreign research teams and strive to conduct research on original technology for the development of next-generation diagnostic techniques. |
|||



| Address | 16th Floor, Posco P&S Tower Teheran-ro 134, Gangnam-gu Seoul 06234 Korea | ||
|---|---|---|---|
| Telephone | +82-2-2015-771 | Fax | +82-2-2015-7900 |
| Website | www.oxfordimmunotec.com | contact-kr@oxfordimmunotec.com | |
| We are a global, high-growth diagnostics company committed to improving patient care by providing advanced, innovative tests in the field of immunology. Our proprietary T-SPOT® technology platform allows us to measure the responses of specific immune cells, known as T cells, to inform the diagnosis, prognosis and monitoring of patients with immunologically controlled diseases. Our first product is the T-SPOT.TB test, which is used to test for tuberculosis infection, and approved for sale in over 50 countries, including the United States and Europe, as well as Korea, Japan and China. The T-SPOT.CMV tests, obtained a CE mark approval in 2015, is our second pipeline focused on the transplantation market. Overall, we have six active development programs, each of which leverages our T cell and innate immune measuring technology. We are headquartered near Oxford, UK and in Marlborough, MA. |
|||

| Address | 5th Floor, Seoul Square 416, Hangang-daero, Jung-Gu, Seoul, Korea | ||
|---|---|---|---|
| Telephone | +82-2-2626-5788 | Fax | |
| Website | www.qiagen.com | heeyoung.lee@qiagen.com | |
| QIAGEN is the leading global provider of Sample to Insight solutions to transform biological materials into valuable molecular insights. QIAGEN sample technologies isolate and process DNA, RNA and proteins from blood, tissue and other materials. Assay technologies make these biomolecules visible and ready for analysis. Bioinformatics software and knowledge bases interpret data to report relevant, actionable insights. Automation solutions tie these together in seamless and cost-effective molecular testing workflows. QIAGEN provides these workflows to more than 500,000 customers around the world in Molecular Diagnostics (human healthcare), Applied Testing (forensics, veterinary testing and food safety), Pharma (pharmaceutical and biotechnology companies) and Academia (life sciences research). Further information can be found at http://www.qiagen.com. |
|||



| Address | 5F, 88, World Cup-ro, Mapo-gu, Seoul, Korea | ||
|---|---|---|---|
| Telephone | +82-2-461-3613 | Fax | +82-2-461-3615 |
| Website | http://www.arkray.co.rk | sdlee@arkray.co.jp | |
| “For all the people around the world to keep their smile.” That is our, ARKRAY's, wish. Since its founding in 1960, we have not only been a pioneer but also continue to focus on diagnostic testing equipment including diabetes testing devices, fully automated urine analyzers, gene analyzers and POCT. Based on our broad network of 22 main offices in 12 countries, we are committed to the principle of local production for local consumption from research and development to production and sales. We will apply the technology and experience we have accrued over the past half century and continue to take on new challenges so that we can answer the diverse needs of our customers whilst maintaining both their health and happiness. |
|||
| Address | Im sung Bldg. NonHyeonro 64-gil 4, Gangnamgu, Seoul, 06231, Korea | ||
|---|---|---|---|
| Telephone | +82-2-3404-3700 | Fax | +82-3404-3785 |
| Website | www.bd.com | ||
| BD is a global medical technology company that is advancing the world of health by improving medical discovery, diagnostics and the delivery of care. The company provides innovative solutions that help advance medical research and genomics, enhance the diagnosis of infectious disease and cancer, improve medication management, promote infection prevention, equip surgical and interventional procedures, and support the management of diabetes. BD has nearly 50,000 associates across 50 countries who work with customers and partners to help enhance outcomes, lower health care delivery costs, increase efficiencies, improve health care safety and expand access to health. bd.com. |
|||
| Address | 8-11 Munpyeongseo-ro, Daedeok-gu, Daejeon, 34302, Korea | ||
|---|---|---|---|
| Telephone | +82-42-930-8777 | Fax | +82-42-930-8600 |
| Website | www.bioneer.com | sales@bioneer.com | |
| Established in 1992, Bioneer Corporation is the first and currently leading biotech company in South Korea. We have over 20 years of experience in R&D, and approximately 440 patents applied and registered. Bioneer has accumulated vast number of proprietary technology and developmental capability to venture into the MDx market. We have developed diverse products from state-of-the-art molecular biology products to automated instruments, including novel enzyme technologies, nucleic-acid extraction instruments, thermal cycler, real-time qPCR, MDx workstations and etc. |
|||
| Address | 10F, Hyunjuk Bldg.,114, Yeoksam-ro, Gangnam-gu, Seoul, 135-936, Korea | ||
|---|---|---|---|
| Telephone | +82-2-3473-4460 | Fax | +82-2-3472-7003 |
| Website | http://www.bio-rad.com | Sales.Korea@bio-rad.com | |
| Bio-Rad Laboratories, Inc. develops, manufactures, and markets a broad range of innovative products and solutions for the life science research and clinical diagnostic markets. The company is renowned for its commitment to quality and customer service among university and research institutions, hospitals, public health and commercial laboratories, as well as the biotechnology, pharmaceutical, and food safety industries. Founded in 1952, Bio-Rad is based in Hercules, California, and serves more than 100,000 research and healthcare industry customers through its global network of operations. |
|||
| Address | Wooyoung Technocenter 2F, 144, Achasan-ro, Seongdong-gu, Seoul, 04783, Korea | ||
|---|---|---|---|
| Telephone | +82-2-498-2340 | Fax | +82-2-498-1189 |
| Website | www.biosewoom.com | oksook@biosewoom.com | |
| Biosewoom is the leading company in the development of viral loads in routine diagnostic viruses, HBV, HCV, CMV, EBV, BK… And provide a broad range of kits by real time per, and pcr ssp, sbt for HLA kits. Biosewoom has quality assurance system for the activities of manufacturing, for in vitro diagnostic reagents for the Field for real time pcr reagents with the requirements of the international standards. Biosewoom is committed to take a new leap through the development of continuous investment in R&D of new. Products using up to date technology.
Attentive to the needs of clinical diagnostics, all our products are offered with a commitment of High Quality and Service. |
|||
| Address | #1013, 800, Gukhoe-daero, Yeongdeungpo-gu, Seoul, 07238 (Yeouido-dong, Jinmi Paragon), Korea | ||
|---|---|---|---|
| Telephone | +82-70-4858-0491 | Fax | +82-2-6264-0491 |
| Website | www.euroimmun.com | euroimmun@euroimmun.com.sg | |
| EUROIMMUN KOREA, Inc., a subsidiary of EUROIMMUN, is a world leader in the field of medical diagnostics. EUROIMMUN established itself as an industry innovator with its unique IFA BIOCHIP Mosaic technology And has successfully introduced new technologies such as ELISA, Western & Immunoblot assays since its inception in 1987. The company’s global success is attributed to its strategic product development in which designer antigens, as well as patented and licensed technologies, play an important role. EUROIMMUN is consistently expanding its product offerings with new assays to aid in the detection of antibodies associated with autoimmune diseases, infectious diseases, and allergy. |
|||
| Address | B-209, 201, Songpa-daero, Songpa-gu, Seoul, Korea | ||
|---|---|---|---|
| Telephone | +82-2-3432-8555 | Fax | +82-2-3432-8556 |
| Website | www.gene-x.co.kr | minho.kim@gene-x.co.kr | |
| GeneX, established in 2013, provides innovative products from Cepheid as exclusive distributor in Korea. It introduces products related to molecular diagnostics and IVD based on Real-time PCR to medical institutions. |
|||
| Address | 107, Ihyeon-ro 30beon-gil, Giheung-gu, Yongin-si, Gyeonggi-do, 16924, Korea | ||
|---|---|---|---|
| Telephone | +82-31-260-0607 | Fax | +82-31-260-9232 |
| Website | www.gclabs.co.kr/eng | tysin@greencross.com | |
| Green Cross Laboratories (GC Labs) is a Korea’s leading clinical laboratory which provides clinical and anatomic pathology reference testing. GC Labs offer more than 4,000 tests and test combinations, ranging from routine tests to highly esoteric molecular and genetic assays. We continue to acquire the latest technology and machines for clinical testing with strong and open partnerships with world-class laboratories and institutions. With our clients, GC Labs will continue to expand beyond Korea to become a world-leading reference laboratory. |
|||
| Address | 14F KTB Building 66 Yeoidaero Yeoungdeungpo-gu, Seoul, Korea | ||
|---|---|---|---|
| Telephone | +82-2-740-5300 | Fax | +82-2-786-8368 |
| Website | www.illumina.com | jkwon@illumina.com | |
| Explore How Genomics is Impacting Human Health
Advancements in our understanding of genetics have the potential to change the practice of medicine and enable genomics-based healthcare. With streamlined workflows and advanced informatics, Illumina sequencing and array technologies and analysis services are allowing you to explore the genome more than ever before. Together, we can address healthcare in ways never before imagined. Visit the Illumina booth to find out how.
|
|||
| Address | 43, Banpo-daero 28-gil, Seocho-gu, Seoul, 06646, Korea | ||
|---|---|---|---|
| Telephone | +82-2-916-6191 | Fax | +82-2-942-2514 |
| Website | www.i-sens.com | cs1@i-sens.com | |
| From its start in 2000 in electrochemistry, i-SENS has successfully developed blood glucose monitoring systems, electrolyte, blood gas and HbA1c analyzers and launched those products successfully in places from its home market in Korea to worldwide markets including the US, Japan, Europe and many others. i-SENS’ POCT analyzers, i-Smart and A1Care, provide simple, fast and reliable test results for healthcare professionals. |
|||
| Address | 2477, Nambusunhwan-ro, Seocho-gu, Seoul, Korea | ||
|---|---|---|---|
| Telephone | +82-2-2109-7830 | Fax | +82-2-852-1984 |
| Website | www.jw-bioscience.co.kr | Web@jw-bioscience.co.kr | |
| JW Bioscience, which is a business firm of JW Group with 70 years of uninterrupted history, is a company specialized in diagnostic and medical devices. Based on our partnerships with the leading medical device manufacturers overseas, we supply medical equipment necessary for every stage of medical services from diagnosis to treatment. We offer operating, examination and obstetric delivery tables as well as automated biochemistry analyzers and automated immunoassay analyzers that are employed at the departments of Laboratory Medicine and Diagnostic Pathology and Blood Collection Lab. JW Bioscience has been expanding its more independent business area by giving a spur to the supply of diagnostic equipment and reagents as well as strengthening R & D capacity. |
|||
| Address | 4F, 508 Teheran-ro, Gangnam-gu, Seoul, Korea | ||
|---|---|---|---|
| Telephone | +82-2-2185-3840 | Fax | |
| Website | www.merckgroup.com | ||
| From drug discovery and development to manufacturing and diagnostics, we are dedicated to solving the toughest problems in life science by collaborating with the global scientific community. Our 300,000 products range from lab water systems to gene editing tools, antibodies, cell lines and end-to-end systems to manufacture drugs. We work closely with our customers from academia, biotech and pharma to help deliver the promise of their work better, faster and safer. Our solutions enable new platforms for drug screening, disease modeling and gene medicine manufacturing, ultimately helping the life science community fulfill the promise of gene therapy, more effectively and efficiently. Together we are driving towards a healthier future – and better outcomes for all. |
|||
| Address | 9th Floor, 18, Teheran-ro 10-gil, Gangnam-gu, Korea | ||
|---|---|---|---|
| Telephone | +82-2-568-8040 | Fax | +82-2-568-8042 |
| Website | www.mindray.com | sonia.oh@mindray.com | |
| Mindray is the only medical device company to be listed in the 100 global challengers in 2016 announced by the Boston Consulting Group. Being one of the global top 5 manufacturers in hematology, we are proud to provide our extensive product portfolio and well established sales & service network for customers worldwide. The accuracy and efficiency of our cellular analysis system also perfectly meets lab test needs for up to thousands of outpatients on daily basis. |
|||
| Address | (Twincity Namsan, Dongjadong), 3F/ 366, Hangang-daero, Yongsan-gu, Seoul, Korea | ||
|---|---|---|---|
| Telephone | +82-2-6222-3535 | Fax | +82-2-6222-3599 |
| Website | www.orthoclinical.com | hjung20@orthoclinical.com | |
| Harnessing the power of reimagination, It’s how Ortho Clinical Diagnostics has been transforming in vitro diagnostics for more than 75 years. It’s made us a trusted partner of hospitals, hospital networks, blood banks, and labs around the world. And today it’s empowering us to advise our customers as they prepare for what’s next. From our earliest work in blood typing to the latest developments in laboratory systems, we’ve pioneered life-impacting advances. Scientific advances that have helped medical professionals diagnose conditions early and make better-informed treatment decisions. Our legacy inspires us every day and continues to drive us forward. |
|||
| Address | 320, Cheonho-daero, Seongdong-gu, Seoul, Korea | ||
|---|---|---|---|
| Telephone | +82-1566-6500 | Fax | +82-2-3394-6503 |
| Website | www.seegenemedical.com/eng | kys16860@mf.seegene.com | |
| Seegene Medical Foundation is a global leading diagnostic testing medical institution that provides over 4,000 test services for clinical practice and research in all fields including diagnostic test medicine, molecular diagnostic test, pathology test, and research test in nationwide hospitals and clinics through more than 600 employees, including the best specialists, masters graduates, doctors, and clinical pathologists in Korea to contribute to human health and happiness through accurate disease testing and innovative research and development. |
|||
| Address | 2F, Hangil Bldg., 3-11, Ogeum-ro 13-gil, Songpa-gu, Seoul, 138-828, Korea | ||
|---|---|---|---|
| Telephone | +82-2240-7040 | Fax | +82-2-2240-7099 |
| Website | www.sgmedical.kr | info@sgmedical.kr | |
| SG Medical, Inc., provides solution in clinical IVD market by trusting partnership with top global companies. As sole distributor of Hitachi - Clinical Chemistry Analyzer, Shino-test – Clinical chemistry reagnet, Christie Medical Holdings – VeinViewer and distributor of Sekisui – clinical chemistry reagent, we carry unique strength in clinical diagnostics market. Our future vision toward developing new items and broadening clinical IVD business will lead us to become the No. 1 global healthcare company. |
|||
| Address | Thermo Fisher Scientific, 12F Suseo Office Building, 281 Gwangpyeong-ro, Gangnam-gu, Seoul, Korea | ||
|---|---|---|---|
| Telephone | +82-2-2023-0600 | Fax | |
| Website | www.thermofisher.com | ||
| Thermo Fisher Scientific Inc. is the world leader in serving science, with revenues of $17 billion and approximately 50,000 employees in 50 countries. Our Mission is to enable our customers to make the world healthier, cleaner and safer. We help our customers accelerate life sciences research, solve complex analytical challenges, improve patient diagnostics and increase laboratory productivity. Through our premier brands – Thermo Scientific, Applied Biosystems, Invitrogen, Fisher Scientific and Unity Lab Services – we offer an unmatched combination of innovative technologies, purchasing convenience and comprehensive support. |
|||
| Address | 16, Magokjungang 8-ro, 1-gil, Gangseo-gu, Seoul, 07795, Korea | ||
|---|---|---|---|
| Telephone | +82-2-3660-6900 | Fax | +82-2-3660-6990 |
| Website | www.wellsbio.net | info@wellsbio.net | |
| WELLS BIO, Inc. specializes in the research, development, and production of in vitro diagnostic tests based on immunochemical, biochemical, and molecular diagnostic technology for early diagnosis of diseases.
Having Access Bio, Inc. as sister company, WELLS BIO, Inc. has outstanding manufacturing capabilities for rapid test kits, molecular diagnostic kits and compact biosensor analyzers and strips that have been proven to have excellent quality in terms of specificity and sensitivity through its clinical studies.
|
|||
| Address | #1101, Hi Brand Bldg., (Living Kwan), Maeheon-ro 16, Seocho-gu, Seoul, 06771, Korea | ||
|---|---|---|---|
| Telephone | +82-1899-9217 | Fax | +82-2-2155-0571 |
| Website | kr.werfen.com | werfenkorea@werfen.com | |
| We are pioneers and developers of IVD testing solutions. We provide high quality systems, reagents and software to labs and hospitals around the world. We strive to enhance care and improve the lives of patients, each and every day. Werfen has become a global leader, thanks to our dedication to Research & Development and a targeted acquisition strategy. Through the years, allowing us to focus on the long-term, and to grow our R&D investment by 10% annually. We are Instrumentation Laboratory, Inova Diagnostics, Biokit and Systelab. We are Werfen. |
|||
| Address | #7803, 140, Beolmal-ro, Dongan-gu, Anyang-si, Gyeonggi-do, 14057, Korea | ||
|---|---|---|---|
| Telephone | +82-31-425-3460 | Fax | +82-31-425-3466 |
| Website | www.wisemeditech.com | wise9@wisemeditech.com | |
| From Wisemeditech started in order to develop new creative products and lead market change in diagnosis field. Wisemeditech deal with medical instruments, diagnosis reagents and vacuum tubes, and also has developed the hospital blood glucose meter named “wisecheck” in home market for the first time in 2012. Recently, Wisemeditech has developed pre-analytical automation system, such as automation tube sorting system named “wise-ATS2000”. Wisemeditech’s future vision toward developing new items and expanding clinical IVD business will make convenient and safe laboratory. |
|||
| Address | AICT Bldg., A, 11F, 145 Gwanggyo-ro, Youngtong-gu, Suwon, 443-270, Korea | ||
|---|---|---|---|
| Telephone | +82-31-888-9596 | Fax | +82-31-888-9095 |
| Website | http://www.astams.com | asta@astams.com | |
| ASTA is a technology-based company that develops and manufactures analytical instruments, equipment for sample preparations, and supplies. In particular, ASTA focuses on the development of MALDI-TOF MS (Matrix Assisted Laser Desorption Ionization Time-of-Flight Mass Spectrometer), its applications and supplies including MALDI-plates, matrix spotter and microwave based sample digestion system. Recently, MALDI-TOF has been introduced and widely used in the medical field as a diagnostic technology. ASTA has been developing various MALDI-TOF based medical applications such as cancer diagnosis and micro-organism identification. The glycan pattern analysis of serum proteins by MALDI-TOF, which is a ASTA owned cutting-edge technology, has successfully applied to early diagnosis of ovarian cancer and expanding its applications to other cancers such as breast cancer. In addition, MALDI-TOF based micro-organism identification system has been launched by ASTA and expanding its usages to medical field, agricultural industry and food industry etc. |
|||
| Address | SNUH Health Care Innovation Park 6F/1F, 172, Dolma-ro, Bundang-gu, Seongnam-si, Gyeonggi-do, Korea | ||
|---|---|---|---|
| Telephone | +82-31-8017-8114 | Fax | +82-31-8017-8124 |
| Website | www.atgen.co.kr | info@atgen.co.kr | |
| We always aim to do our part in improving everyone's quality of life, whether by providing novel diagnostic tests, developing new therapies, or providing the research community with quality proteins and antibodies that are crucial for life science advancements. Based on the core SP fusion technology (Stable-peptide), ATGen’s monoclonal antibodies are manufactured in house and this led ATGen to expand our field of expertize. ATGen started expanding into the diagnostic market by developing an ELISA test for influenza in 2009, then further developing an innovative world’s first NK cell function test called NK Vue. Based on this, ATGen also launched NK mouse kit for research use and NK Puppy kit for pet dogs’ immune management. |
|||
| Address | #913, Kolonvillant 1, 30, Digital-ro 32-gil, Guro-gu, 08390, Korea | ||
|---|---|---|---|
| Telephone | +82-2-6949-1751 | Fax | +82-2-864-9954 |
| Website | http://biois.co.kr/ | marketing@biois.co.kr | |
| Biois has been researching and developing products and technologies that can diagnose cancer and chronic diseases with just a drop of blood and/or body fluids. Our core patented technology is ideal for biomarker discovery and development of new drugs, which will help patients suffering from various cancers and chronic diseases. In addition, we have built a specialized infrastructure in the field of bioinformatics with various application technologies using biomarker development and human
derived material analysis technology, and are conducting clinical trials, diagnosis services, and new drug development through foreign renowned hospitals and research institutes. Biois has been reading new trends to prepare for the rapidly changing 21C and strives to pursue new technologies |
|||
| Address | 2-2-ho, 56, Soyanggang-ro, Chuncheon-si, Gangwon-do, 24232, Korea | ||
|---|---|---|---|
| Telephone | +82-33-258-6097 | Fax | +82-33-258-6099 |
| Website | www.bmtchip.com | wsyoo@bmtchip.com | |
| Biometrix Technology Inc. (BMT), is the leading company of biochip industry and specialized in DNA testing products. BMT is a biochip company that specializes in making oligonucleotide DNA Chips and Genotyping kits for early detection of viral or bacterial infections. BMT has raised a fund from Korean venture investment syndicates and spent its energy in pursuing the major R&D in the field of DNA Testing. Focusing on new technologies, new markets for near patient diagnostic products, and new attitudes of patients and healthcare providers, BMT is going forward into the fastest growing sector of worldwide diagnostic industry whose products are transforming the practice of medicine.
|
|||
| Address | 43 Geodudanji 1-gil, Dongnae-myeon, Chuncheon-si, Gangwon-do, 24398, Korea | ||
|---|---|---|---|
| Telephone | +82-33-243-1400 | Fax | +82-33-243-9373 |
| Website | www.boditech.co.kr | sales@boditech.co.kr | |
| Boditech Med Inc. has been developing products for in-vitro diagnostics and diagnostic reagents for the past 20 years by pursuing the value of a company called "Respect for Life". In particular, about 25,000 units of ichroma ™ readers are installed and operated world wide, and ichroma™ CRP (C-Reactive Protein) has secured its largest market share in China, showing a unique competitiveness.
Boditech Med Inc. is leading the way in exploring new markets through the automation of on-site diagnosed immunodiagnostic devices and the development of new high-sensitive diagnostic reagents. We have built up our reputation successfully by carrying out various national R & D projects based on solid technology, experience, and human resources accumulated over the years.
In addition, Boditech Med Inc. is developing a smart production system that integrates digital technology and IT technology to flexibly manage the production scale. All employees of Boditech Med Inc. is making every effort with pride that we are the top leader of BT-IT convergence industry in Korea.
|
|||
| Address | 64 Cheonhodaero, Dongdaemun-gu, Seoul, Korea | ||
|---|---|---|---|
| Telephone | +82-2-2173-7207 | Fax | +82-2-927-4946 |
| Website | http://en.donga-st.com | sangchuli@donga.co.kr | |
| Dong-A ST focuses on ethical drugs, such as domestically developed new drugs like Stillen, Zydena, and Motilitone, medical devices, diagnosis, and oversea businesses. Our vision is to provide "Total health care solution" from early accurate diagnosis to complete treatment. |
|||
| Address | 30, Seolleung-ro 100-gil, Gangnam-gu, Seoul, Korea | ||
|---|---|---|---|
| Telephone | +82-2-556-4579 | Fax | +82-2-556-4581 |
| Website | www.dbpoc.co.kr | dbpoc@dbpoc.co.kr | |
| Dongbang POC Co., Ltd. was separated in 2006 as an independent company from Dongbang Healthcare Co., Ltd which has been in medical device industry since 1979. Dongbang POC specializes in Clinical Laboratory medical devices and is currently representing Nova Biomedical(blood gas/electrolyte analyzers) and Arkray (dry chemistry analyzers). |
|||
| Address | 291 Harmony-ro, Yeonsu-gu, Inchon, 22014, Korea | ||
|---|---|---|---|
| Telephone | +82-32-210-2100 | Fax | +82-32-210-2233 |
| Website | www.eonelab.co.kr | kmh6015@eonelab.co.kr | |
| Founded in 1983, Eone Laboratories has dedicated to improving health care system by introducing a number of leading-edge testing techniques. With a national sales network, we provide accurate and timely results of laboratory tests through rigorous quality control. Based on our knowledge and experience accumulated over the years, Eone will contribite to excellent patient care by providing comprehensive, high quality laboratory testing. |
|||
| Address | 201-22, Geomjun 2-gil, Eunhyeon-myeon, Yangju-si, Gyeonggi-do, Korea | ||
|---|---|---|---|
| Telephone | +82-31-858-6344 | Fax | +82-31-868-6344 |
| Website | www.gsmedical.co.kr | giantstar@hanmail.net | |
| GMS is very specialized with manufacturing blood vaccine storage devices. We have long history and much experience regarding such devices. It means that we know how to install, how often need to visit ultimate place, how to handle emergency status. We are tiny but working 24hrs on every single day in Korea. |
|||
| Address | 8, Osongsaengmyeong 5-ro, Osong-eup, Heungdeok-gu, Cheongju-si, Chungcheongbuk-do, 28161, Korea | ||
|---|---|---|---|
| Telephone | +82-43-229-6200 | Fax | +82-43-229-6210 |
| Website | www.hanlab.co.kr | hanlab@hanlab.co.kr | |
| HANLAB CORPORATION, which has been founded in 1993, has developed, manufactured and sold products and reagents to use in laboratory medicine with respect for life and a lot of experiences in the field of laboratory medicine. As a company focused on R&D, HANLAB has 30% of employee who are belonged to R&D Laboratory and has developed the world first ABC(Automatic Balancing Centrifuge), LEPAS(Lab Efficient Pretreatment Automation System, and RMS(Remote Monitoring System), under the brand name 'Labmaster'. Besides, HANLAB has made an earnest effort to be a global healthcare company which contributes medical welfare society by providing innovative and convenient products and solution such as Labmaster. |
|||
| Address | 7F. #B, Korea Bio Park, 700, Daewangpangyo-ro, Bundang-gu, Seongnam-si, Gyeonggi-do, 13488, Korea | ||
|---|---|---|---|
| Telephone | +82-31-628-2100 | Fax | +82-31-628-2108 |
| Website | www.hbi21.com | hbidiag@hbidiag.com | |
| HBI is a biotech company specialized in development and commercialization of multiplex molecular diagnostics, clinical biochemistry and immunology-based diagnostic products. HBI is pursuing a challenging growth strategy by incorporating its platform technologies and strong key opinion-leading clinical network with focus on infectious diseases and new biomarkers related with life-threatening chronic diseases such as cancers and cardiovascular disorders. |
|||
| Address | #201. Jeonpa-ro 88, Dongan-gu, Anyang-si, Gyeonggi-do, Korea | ||
|---|---|---|---|
| Telephone | +82-31-478-8597 | Fax | +82-31-478-8586 |
| Website | www.humasis.com | question@humasis.com | |
| HUMASIS strives to develop, manufacture, and market IVD for the detection of cardiac markers, fertility hormones, infectious diseases, and tumor markers in rapid tests for home and laboratory use. New quantitative POCT analyzer introduces its new parameters regularly to cover the constantly expanding range of applications. HUMASIS has reached more than 50 countries of distribution worldwide. |
|||
| Address | 4, 5, 6F, Manseongjae Bldg, 17, Yangjae-daero 85 gil, Gangdong-Gu, Seoul, 05408, Korea | ||
|---|---|---|---|
| Telephone | +82-2-404-7990 | Fax | +82-2-404-7992 |
| Website | www.In-Sol.co.kr | wjk@in-sol.co.kr | |
| Insol supplies POCT(Point of care Test) Oraquick Adv, Oraquick HCV to the emergency room, providing HIV and HCV antibody test results of percutaneous injuries within 20 minutes. This offers an infection control, protection of cross-contamination between the patients, preventing infection of medical staff in ER. |
|||
| Address | 144, Donggureungro 395 beongil, Guri-si, Gyeonggi-do, Korea | ||
|---|---|---|---|
| Telephone | +82-31-570-5700 | Fax | +82-31-570-5799 |
| Website | www.kmhealthcare.co.kr | cmoh@kmbiz.com | |
| KM Healthcare Corp. was founded in 1995, as the first company introducing disposable surgical gowns and drapes in Korea. KM Healthcare is also producing and supplying medical supplies. We will make continuous efforts to develop and expand our products line and business. As a professional company to provide total solution for medical staffs and patients, KM Healthcare promises to realize the real partnership with you. |
|||
| Address | C-1101, 168 Gasandigital-ro, Geumcheon-gu, 08507, Seoul, Korea | ||
|---|---|---|---|
| Telephone | +82-2-2026-2150 | Fax | +82-2-2026-2155 |
| Website | www.kogene.co.kr | kogene@kogene.co.kr | |
| KogeneBiotech is an on-demand molecular diagnostic company which has 16 years’ experience in the field of the nucleic acid-based test kit. Based on expertise and active technical support, we provide our customer with the state-of–the art Real-time PCR kits of the wide range of parameters which can cover clinical dx, food safety test, and animal health. In 2002, KogeneBiotech has become the first company, who adopted the Real-time PCR technology in Korea. In 2014, KogeneBiotech were earned the industrial service medal of science and technology for Food safety from the Korea government. Following that, Kogene's method and kit were selected as a monitoring system standard method by government. For MERS-CoV and MTB kits, we were studied with medical institutions and researcher and published journal or paper constantly. Especially, MERS-CoV kit was developed in 2014 and it has been used to the standard method in 2015 Korea MERS outbreak. |
|||
| Address | RM. 1104 Uspace 1 A-dong, 660 Daewangpangyo-ro, Bundang-gu, Seongnam-si, Gyeonggido, Korea | ||
|---|---|---|---|
| Telephone | +82-31-628-1900 | Fax | +82-31-628-1905 |
| Website | www.ki-med.co.kr | seunghee@kyungilhitec.co.kr | |
| Since its founding in 1988, Kyungil Medical has continuously discovered leading and useful products and has grown with customers through constant investment. Kyungil Medical’s efforts to satisfy the customers with the honest management, excellent products, and sincere services has been continuing ever since. We will continue to take responsibility and role to lead the diagnostic industry, aspiring to bring better health and a brighter future for hospitals in Korea. |
|||
| Address | Unit 1206, Lemmi Centre, 50 Hoi Yuen Road, Kwun Tong, Kowloon, Hong Kong | ||
|---|---|---|---|
| Telephone | +852-2234-5680 | Fax | +852-2234-5766 |
| Website | www.luminexcorp.com | sshin@luminexcorp.com | |
| Luminex is founded in 1995 and Headquartered is located in Austin. Luminex is rapid growth with offices around the world based on serve both the Life Science Research and Diagnostics Markets. Luminex have been partnership with 60 companies related of Life science and provided bead material and instrument for multiplex analysis. Luminex have developed IVD product as like GPP and RPP product in focus on multiplex technology by itself and tried to help accurate and rapid diagnosis. |
|||
| Address | Evropska 655/116, 160 00, Prague 6, Czech rep., Europe | ||
|---|---|---|---|
| Telephone | +420-778-767-510 | Fax | |
| Website | www.mediware.cz | potucek@mediware.cz | |
| MEDIWARE, Inc. is a software company focused on health services. We are the leading company in the area of the TDM software on the world’s market. Our solutions help in hospitals, medical and research institutions and they provide better health care. We offer the platform to everybody who participates in personalized health care or drug research & development.
|
|||
| Address | 12F, 5, Digital-ro 26 gil, Guro-gu, Seoul, 08389, Korea | ||
|---|---|---|---|
| Telephone | +82-2-6220-7867 | Fax | +82-2-6220-7999 |
| Website | www.nanoentek.com | ivdst@nanoentek.com | |
| NanoEnTek has key technology of nano scale Bio-MEMS that organizationally combined microelectromechanical system (MEMS) and biotechnology, which is called technology of the 21st century. Based on the technology, it is expanding its business to life science experiment equipment, medical in-vitro diagnostic device and related solution development. There are about 100 patents of nano convergence technology that were applied and registered. |
|||
| Address | 1104, Hanhwa Bizmetro, 242, Digital-ro, Guro-gu, Seoul, Korea | ||
|---|---|---|---|
| Telephone | +82-2-867-9798 | Fax | +82-2-883-9784 |
| Website | www.ngenebio.com | business@ngenebio.com | |
| NGeneBio was founded in October 2015 as a joint venture between Genecurix and Korea Telecom, develops in vitro diagnostics (IVD)/companion diagnostics (CDx) products and bioinformatics software (SW) with cutting-of-edge technologies including next generation sequencing (NGS). The company launched NGS-based hereditary breast/ovarian cancer panel (BRCAaccuTest®) with clinical analysis SW (NGeneAnalysisTM) and received CE-IVD mark in Jun/2017. NGS panels for solid tumor/hematologic malignancies and BRCA PARP inhibitor CDx test will be launched, inclusive of clinical data analysis SW platform. In support of laboratory medicine community, our mission is to provide clinically validated NGS-IVD/CDx products/services with convergence of innovative biotechnology and bioinformatics. |
|||
| Address | 132, Anyangcheondong-ro, Dongan-gu, Anyang-si, Gyeonggi-do, 14040, Korea | ||
|---|---|---|---|
| Telephone | +82-31-460-9937 | Fax | +82-31-460-9933 |
| Website | www.osanghc.com/en | haroldjung@osanghc.com | |
| Devoted since inception to the development of diagnostic biosensors for blood glucose measurement, OSANG Healthcare envisions medical devices becoming as commonplace as home appliances, easily measuring all diseases across the globe as “Technology for Human.” Today, we export the world’s best diagnostic biosensors for blood glucose, HbA1c and cholesterol to more than 110 countries the world over, in our drive to become the leading researcher and developer of diagnostic sensors for heart disease and cancer, and of remote diagnosis systems. |
|||
| Address | 2F, 172 Janhan-ro, Dongdaemun-gu, Seoul, 02522, Korea | ||
|---|---|---|---|
| Telephone | +82-2-2294-1614 | Fax | +82-2-2294-1655 |
| Website | www.pharmode.co.kr | pharmode@hanmail.net | |
| PHARMODE CO. Ltd. is a global medical technology company that specialize in the development and sale of Scanning Capillary Method Viscometer. (Hemovister®) We focus on supporting physicians so that they can use blood viscosity as one of clinical diagnostic, prognostic and therapeutic tools. |
|||
| Address | 15Fl, 741, Yeongdong-daero, Gangnam-gu, Seoul, 06071, Korea | ||
|---|---|---|---|
| Telephone | +82-2-2056-9300 | Fax | +82-2-512-2991 |
| Website | www.radiometer.com | info@radiometer.kr | |
| Radiometer develops, manufactures and markets solutions for blood sampling, blood gas analysis, transcutaneous monitoring, immunoassay testing and related IT management systems under the ABL, AQT, TCM, RADIANCE, AQURE, PICO, CLINITUBES and QUALICHECK brand names. Founded in 1935 and headquartered in Copenhagen, Denmark, Radiometer was a pioneer in blood gas testing, introducing the world’s first commercially available blood gas analyzer in 1954. Today, Radiometer’s products and solutions are used in hospitals, clinics and laboratories in over 130 countries, to provide information on the most critical parameters in acute care testing. |
|||
| Address | 2F, 25 Heungan-daero, Gunpo-si, Gyeonggi-do, Korea | ||
|---|---|---|---|
| Telephone | +82-31-427-4677 | Fax | +82-31-427-4678 |
| Website | www.rapigen-inc.com | rnd@rapigen-inc.com | |
| RapiGEN Inc. keeps growing in the diagnostic industry using our leading-edge technology, the Black Gold Particle. It can be represent the Dual Color System which is a step ahead of the current rapid screening test methods and engenders visibly advanced results in the test performance, the sensitivity and specificity. We are actively broadening our market share in the diagnostic industry with our high quality products which were already proved from our various royal customers all over the world. Through continuous efforts to develop and produce impeccable and more innovative high quality products, our ultimate target is to make the betterment of the world and supply our cutting-edge products with affordable price. |
|||
| Address | 285, Digital-ro, Guro-gu, Seoul, Korea | ||
|---|---|---|---|
| Telephone | +82-2-6959-0115 | Fax | +82-70-8220-6616 |
| Website | www.r-biopharm.com | sykwon@r-biopharm.co.kr | |
| R-Biopahrm is a leading developer of test solutions for clinical diagnostics. Since 1988, we have developed innovative products and pioneering solutions of the highest quality, safety and efficiency in Darmstadt. We are leaders in the stool diagnostics, especially for a rapid detection of noroviruses. Our validated test systems for serological infection, allergy and molecular diagnostics and gastroenterology are generally characterized by a simple handling. |
|||
| Address | #908, 148 Sagimakgol-ro, Jungwon-gu, Seongnam-si, Gyeonggi-do, 13207, Korea | ||
|---|---|---|---|
| Telephone | +82-2-576-9331 | Fax | +82-2-814-2229 |
| Website | www.samilpt.co.kr | choisook@samilpt.co.kr | |
| Since 1967, along with the Korean medical diagnostics, the company has grown to be the leader of the market in supplying medical products. Samil P&T offers customers a variety range of high quality reagent and analytical instruments as well as reliable technical service to enable integrated clinical laboratory workflow solution possible. Samil Bio-science will provide a complete portfolio of research laboratory equipment, regents, chemical supplies with high quality technical service used in scientific research, healthcare and science education. |
|||
| Address | 57, Baumoe-ro 41-gil, Seocho-gu, Seoul, Korea | ||
|---|---|---|---|
| Telephone | +82-2-3497-5100 | Fax | +82-2-3497-5169 |
| Website | www.smlab.co.kr | smlpr@smlab.co.kr | |
| Samkwang Medical Laboratories(SML) is a leading provider of laboratory testing, helping healthcare providers diagnose, treat, monitor and prevent disease in patients. At every step in the testing process, our goal is to provide caring, efficient, reliable, and high-quality services that contribute to enhanced patient care. SML will continue to lead the industry with healthcare innovations that provide better health with diagnostic insights. |
|||
| Address | 13, Heungdeok 1-ro, Giheung-gu, Yongin-si, Gyeonggi-do, Korea | ||
|---|---|---|---|
| Telephone | +82-1800-0119 | Fax | +82-2-790-6509 |
| Website | www.scllab.co.kr | ||
| Seoul Clinical Laboratories (SCL), a clinical diagnostic laboratory founded in 1983, is the first referral laboratory in Korea to receive accreditation from the CAP. By endeavoring to fulfill its core values, ‘Quality, Service and Research’, SCL has developed into the largest nationwide laboratory with the best reputation in the reference laboratory field in Korea. SCL provides a wide variety of specialized laboratory assays and cutting edge research tests to over 4,500 medical institutions nationwide. SCL intends to increase its presence as the world class reference laboratory by providing precise test results and high quality customer-oriented services to medical practitioners throughout the world, as well as throughout Korea. |
|||
| Address | C-5th Floor. Digital Empire Building, Yeongtong-dong, Yeongtong-gu, Suwon-si, Gyeonggi-do, Korea | ||
|---|---|---|---|
| Telephone | +82-31-300-0440 | Fax | +82-31-300-0458 |
| Website | www.sdbiosensor.com | sdb@sdbiosensor.com | |
| SD BIOSENSOR is a company specialized in in-vitro diagnostics founded with the goal of contributing to improving the quality of life through fast and accurate diagnosis of disease. We have become capable of providing diagnostic products of excellent quality by developing fluorescence immunoassay products that can perform qualitative as well as quantitative analyses through immunoassay methods using antigen and antibody responses, and we will not stop here but keep striving to become the No. 1 global in-vitro diagnostic company through continuous technological innovations. |
|||
| Address | 12F, Suseo Office Bldg., 281 Gwangpyeong-ro, Gangnam-gu, Seoul, Korea | ||
|---|---|---|---|
| Telephone | +82-2 540-3311 | Fax | +82-2 540-6333 |
| Website | www.seongkohn.co.kr | kcsung@seongkohn.co.kr | |
| SeongKohn Traders’ Corp. is specialized in the instruments, consumables and reagents for the pathology labs as well as providing Digital Solutions including digital(virtual) slide preparation & web-hosting and automated image analysis of IHC stained slides. The company is representing Thermo Fisher Scientific APD, Hologic, Inc., 3DHISTECH Kft., Applied Spectral Imaging, Inc., and Biocartis NV.. |
|||
| Address | (#O-1003,ITECO) 150, Jojeongdaero, Hanam-Si, Gyeonggi-do,12930, Korea | ||
|---|---|---|---|
| Telephone | +82-31-790-1070 | Fax | +82-31-790-1071 |
| Website | www.shinmedi.com | mail@shinmedi.com | |
| We are manufacturer of IVD Reagent under KGMP and we developed QC management system, "EasyQC". We have a nationwide sales network as a main distributor of WAKO and FURUNO. We deliver the quality and the satisfaction to the customers. |
|||
| Address | 14, Bongeunsa-ro 43gil, Gangnam-gu, Seoul, 06103, Korea | ||
|---|---|---|---|
| Telephone | +82-2-546-4081 | Fax | +82-2-546-4370 |
| Website | www.sy-diagnostics.com | admin@sy-diagnostics.com | |
| Shinyang Chemical Co., Ltd, we have continued to grow in quantity/quality as a specialist for in-vitro diagnostics for over 30 years. A diagnostics field is undergoing change and improvement;
1) we have competitive manufacturing sector with quality improvement skills and research,
2) building cooperative network of companies with domestic and abroad excellent diagnostics business and
3) have talent employees to meet higher than expected customer demand, so we can prepare any challenges in the future.
We will communicate with customer for the reliable partnership and effort to the improvement. |
|||
| Address | 1st Floor,13, Nonhyeon-ro 81-gil, Gangnam-gu, Seoul, Korea | ||
|---|---|---|---|
| Telephone | +82-2-552-1951 | Fax | +82-2-552-1972 |
| Website | mail@ssmedipia.com | ||
| Day in, day out, Stago is committed to enhancing healthcare quality by offering laboratories advanced testing systems and superior services, the fruit of our Haemostasis expertise and know-how (reagents, instruments, disposables and data management). With over 350 marketed products, Stago is a worldwide reference in Haemostasis and a 1st class partner for biomedical laboratories. Specialised in the fields of Haemostasis and Thrombosis, Stago invests in research and innovation to develop new and better performing reagents, systems and solutions. With 50 years of experience, Stago has acquired a charismatic image in Haemostasis and is well recognised among the international scientific world. |
|||
| Address | Babraham Research Campus, Cambridge, CB22 3AT, United Kingdom | ||
|---|---|---|---|
| Telephone | +44-01223-496700 | Fax | +44-1223-496705 |
| Website | Twistdx.co.uk | info@twistdx.co.uk | |
| TwistDx Ltd, based in Cambridge UK, has developed the world’s leading, next generation isothermal DNA amplification technology, Recombinase Polymerase Amplification (RPA), which offers PCR-like sensitivity within 10 minutes of reaction time. RPA replaces PCR with a rapid, isothermal, enzymatic process using a recombinase which pairs oligonucleotide primers to template DNA and a polymerase to synthesize daughter strands. RPA products can be detected by a variety of means including electrophoresis, lateral flow strips or real-time fluorescence detection. |
|||
| Address | Hoju Bldg. 2F, 16, Dongsan-ro 6-gil, Seocho-gu, Seoul, 137-897, Korea | ||
|---|---|---|---|
| Telephone | +82-2-529-7492 | Fax | +82-2-529-7495 |
| Website | www.unionlab.co.kr | hscho@unionlab.co.kr | |
| Since its establishment in 1999, as one of the most capable diagnostic distributors in Korea, UnionLab Inc. has been importing and distributing quality diagnostic products from global manufacturing partners. Our mission is to supply the best in class quality products to our valuable customers by cooperation with excellent diagnostic manufacturers all over the world. Through this creation of values we have been contributing to our customers and to the development of laboratory medicine society of Korea. UnionLab Inc. is representing Diagnostic Grifols for transfusion medicine, Fujirebio-Europe for Neuro diagnostics and human HLA, MP Biomedicals for infectious disease, Hanil Komed for micribiology culture media, bioMerieux for Etest, CerTest Biotec for Rapid infectious diagnosis, MKL for bacterial identification, Copan Floqtechnology for transport medium and swab system, etc. |
|||
| Address | 1113 Building A, Terra Tower 2, 201 Songpa-Daero Songpa-Ku, Seoul, Korea | ||
|---|---|---|---|
| Telephone | +82-2-881-5432 | Fax | +82-2-881-5454 |
| Website | www.woongbee.com | woongbee@woongbee.com | |
|
|
|||
| Address | 76 Seori-ro, Idong-myeon, Cheoin-gu, Yongin-si, Gyeonggido, Korea | ||
|---|---|---|---|
| Telephone | +82-31-329-2000 | Fax | +82-31-329-2002 |
| Website | www.yd-diagnostics.com | admin@yd-diagnostics.com | |
|
|
|||
| Address | 60, Anyangcheondong-ro, Dongan-gu, Anyang-si, Gyeonggi-do, 14042, Korea | ||
|---|---|---|---|
| Telephone | +82-2-1544-1348 | Fax | +82-31-428-8709 |
| Website | www.youngin.com | youngin@youngin.com | |
| Young In Scientific Co., Ltd. Was founded in 1976 has been specializing in science and technology to continuously improve the quality of customers' research by providing advanced precision test and analysis equipment sales and services for customers.
During the last 40 years, we have focused on supplying and servicing Laboratory Analytical System and Medical Device with advanced technologies to various industries and educational organizations for the purpose of research, test and QA/QC.
We are one of the largest distributors of scientific, analytical, medical, clinic, electrochemical, process instruments in the world.
With our commitment to customer support satisfaction and problem-solving, we ensure our outstanding performance in marketing, sales, applications and technical support.
|
|||




